mRNA Drug Impurity Profiling
mRNA Drug Impurity Profiling
The rapid advancement of nucleic acid-based therapeutics, such as mRNA vaccines, represents a significant leap forward in our ability to respond to infectious diseases and develop treatments for a variety of conditions. Leveraging technologies like in vitro transcription (IVT) for mRNA synthesis or circular RNA (circRNA) for vaccine production, we stand at the forefront of a new era in medicine. Rockland supports these efforts with products and services tailored to the unique challenges of mRNA vaccine and therapeutic development.
The Challenge of Impurities
In large-scale production of IVT mRNA and circRNA vaccines, impurities present a significant challenge to both safety and efficacy. These impurities can arise at various stages of the manufacturing process, including both upstream and downstream transcription.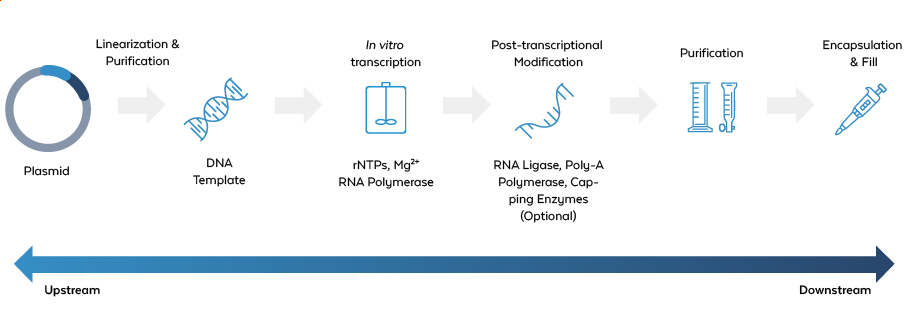
Classes of Impurities
Unreacted Reagents
Components used in the IVT process, such as nucleotides, enzymes (e.g., T7 RNA Polymerase, T4 Ligase), or plasmid DNA, which can remain in the final product.
Byproducts
Undesirable entities like double-stranded RNA (dsRNA), incomplete mRNA fragments, and mRNA aggregates that can arise during synthesis.
Residual Host Cell Components
Contaminants including host cell DNA and RNA that may carry over from the biological systems used in the manufacturing process.
These impurities not only compromise the integrity and purity of the final product but also pose safety risks. Unwanted immune responses can be triggered by these contaminants, leading to adverse effects in patients. Moreover, impurities like aggregates can interact with the mRNA, affecting its stability and function.
mRNA Impurity Detection
Ensuring the purity of mRNA therapeutics is critical for their success. This requires sophisticated analytical tools and strategies for detecting and quantifying impurities throughout the purification process. One of the key technologies is the use of antibodies designed to target and identify specific impurities. These antibodies are invaluable for their consistency and scalability, making them essential for both small-scale research and large-scale manufacturing.
Rockland is an impurity detection expert for the biopharmaceutical community, offering custom and off-the-shelf host cell protein antibodies and assays for over 15 years. With that expertise, we have developed antibodies against common impurities that are a result of large-scale IVT mRNA production. Currently available are antibodies to T7 RNA polymerase and T4 RNA ligase. Stay tuned as we continue to build on this suite of mRNA impurity detection antibodies and contact us if you need custom services while these reagents become available.
| Product | Target Details |
| T7 RNA Polymerase Antibody | T7 RNA polymerase is used in the IVT process for mRNA vaccine production. T7 RNA polymerase synthesizes mRNA from a DNA template. |
| T4 RNA Ligase Antibody | T4 RNA ligase is involved in the production of circRNA vaccines. T4 RNA ligase catalyzes the ligation of RNA molecules to form a covalently closed loop. |