In-Cell Western (ICW) Protocol
- 13 steps
- 6 hours
- 9 reagents required
In-cell Western assays (ICW) also known as In-cell ELISA (ICE) allow researchers a simple and rapid assay method for the detection of biomarkers and signaling proteins in whole cells using antibodies. The protocol described below is for 96-well microtiter plates. Please adjust buffer volumes to account for plates with small or larger well volumes. 384-well plates may be used if desired. This protocol is known to work for the LICOR® Odyssey® Classic, Odyssey® CLx, and Odyssey® Sa Imaging Systems. This protocol may work on other imaging systems that can detect in the near IR spectrum.
Reagents Required
| Product | Preparation |
| 10X Phosphate-Buffered Saline (PBS) | Use sterile deionized water or equivalent for dilution for stock solutions. |
| 10X PBS Fish Gel | Dilute Bovine Serum Albumin (BSA) or non-fat dried milk are not recommended but can be used in some experiments. |
| Blocking Buffer/Antibody Diluent | Prepare 1X PBS Fish Gel |
| Formaldehyde 16% (w/v) | Use fresh, store-opened vials at 4°C in the dark. Dilute to 3.7% in PBS for use. |
| Permeabilization Buffer | Prepare 80 mL 1X PBS + 1.0% Triton X-100 per plate. Triton must be supplied by the user. |
| Primary Antibody | See available primary antibodies |
| Secondary Antibody | See available conjugated secondary antibodies |
| Active Cell Culture | Prepare actively growing cells of interest (see protocol below) |
| 96-Well Culture Plate | Clear- or black-walled plate may be used. A black-walled plate should be used for critical data collection. |
Procedure
-
Grow cells in 96-well or 384-well plates until confluent by standard procedures. Serum-starve cells if necessary and proceed to stimulate or monitor cells.
-
Proceed to fix cells with a 3.7% formaldehyde solution in PBS for 20 minutes at room temperature. Add 150 µL of formaldehyde solution. Incubate on bench top for 20 minutes at ambient temperature. Do not agitate.
-
Discard fixative solution. Blot inverted plate by tapping onto clean paper towel. Gently add 200 µL of permeabilization buffer to each well with multichannel pipette and gently rotate plate for 5 minutes at ambient. Repeat the wash step 5 times.
-
Block cells by adding 150 µL of blocking buffer for 1.5 hours at room temperature with gentle shaking (blocking times up to overnight are acceptable).
Note: Alternative blocking reagents: Rabbit Serum Non-Sterile, Mouse Serum Non-Sterile -
Prepare primary antibody by diluting in antibody dilution buffer. A suggested starting dilution range is 1:50–1:500. Increase the amount of antibody tenfold relative to what is recommended for a conventional Western blot. If performing a double-labeling experiment, combine both primary antibodies as a cocktail to the desired concentration using antibody dilution buffer.
-
Tap out the blocking solution into the sink and blot onto a paper towel. Add diluted primary antibody (total volume 50 µL/well). Cover plate and incubate for 2 hours at room temperature with gentle shaking. If greater sensitivity is desired, continue incubation overnight at 4°C with plate stationary.
-
After primary antibody incubation, rinse plate 4 times with wash buffer for 5 minutes each wash.
Note: In-cell Western allows for simultaneous detection of two primary antibodies. Primary antibodies must be from different host sources, i.e. mouse, rabbit, or goat. The secondary antibodies must discriminate and not cross-react between the hosts of the primary antibody. Alternatively, the primary antibodies must be directly conjugated to a 700 channel and an 800 channel fluorochrome respectively. -
Dilute the detection antibody in dilution buffer or other appropriate blocker. A starting dilution of 1:1000 will work for most basic applications, although dilutions from 1:200–1:2000 are commonly used.
Note: In some cases the dilution may require optimization. Fluorochrome-conjugated antibodies should be shielded from exposure to prolonged periods of direct light. -
Add fluorochrome-conjugated secondary antibody diluted in antibody dilution buffer (total volume 50 µL/well) and incubate for 1 hour at room temperature in the dark with gentle shaking.
Note: For double-labeling, prepare a cocktail of fluorochrome-conjugated anti-mouse and anti-rabbit secondary antibodies in antibody dilution buffer. Each should have a different fluorochrome-dye conjugate where one dye works in the 700 channel and the second dye works in the 800 channel. -
After incubation, rinse the plate 4 times with wash buffer for 5 minutes each and blot dry when finished.
-
Scan the dry plate on LICOR® Odyssey™ or equivalent imaging system according to manufacturer’s directions.
-
If using the Odyssey™ imager, set the device for the appropriate detection channels. The intensity settings should be set for moderate sensitivity. Set both 700 and 800 nm channels to 5. However, if the image shows saturation, repeat the scan with lower intensity setting (i.e., 3 or 1.5). If the signal is very low, the intensity setting may be set higher such as 7 or 8.
-
Resolution settings can be set to medium to obtain a clean result with an acceptable scanning time.
Note: Fixed and stained plates can be stored for 1–3 weeks for future use. To preserve the experiment for a later scan, protect the plate from light by wrapping in a foil pouch (or equivalent). Store the plate at 4°C prior to use.
Procedure for Tubulin Detection as a Positive Control
The following example is a positive control experiment to demonstrate the detection of tubulin. In this experiment, Capan-2 pancreatic cancer cells are grown and seeded into a 96-well ELISA plate and probed for alpha-tubulin.
Capan-2 cells are grown to sub-confluence in a T-150 flask. The cells are trypsinized and then seeded into a 96-well tissue culture plate. Black-walled plates may be used to reduce side scatter.
The recommended seeding density is 0.2 x 106 cells per mL. Allow the cells to grow to confluence (for Capan-2 cells approximately 48 hours). Since alpha-tubulin is cytosolic, the cells are fixed and permeabilized. Permeabilization with Triton X-100 allows for greater antibody penetration into the cell interior. As a negative control, cells in a portion of the plate were not permeabilized.
Note: Permeabilization is not necessary for antibodies that target extracellular protein domains.
- Remove media from wells and gently wash the cells 2 times with 1X PBS by adding 200 µL/well for 5 minutes each at room temperature with gentle shaking.
- Add 150 µL/well 3.7% formaldehyde in 1X PBS. Incubate 20 minutes at room temperature without shaking.
- Remove fixing solution by gentle aspiration using multichannel pipet.
- Wash and permeabilize cells with 200 µL/well of 1X PBS + 0.1% Triton X-100 4 times for 5 minutes each at room temperature with gentle shaking.
- Gently flick out contents of wells between each wash. Periodically check to confirm cells have not detached from the plate.
- Block by adding 150 µL/well of 1X PBS fish gel solution.
- Incubate blocking step for 1.5 hours at room temperature with gentle shaking.
- Wash by adding 200 µL /well 1X PBS + 0.1% Tween-20 4 times for 5 minutes each at room temperature with gentle shaking.
- Add 50 µL /well mouse anti-alpha tubulin primary antibody diluted to 2.5 µg/mL (1:400) in blocking buffer.
- Incubate primary antibody for 2 hours at room temperature with gentle shaking then move to 4°C for overnight incubation without shaking.
- Wash by adding 200 µL/well 1X PBS + 0.1% Tween-20 4 times for 5 minutes each at room temperature with gentle shaking.
- Add 50 µL/well Anti-MOUSE IgG (H&L) Antibody Dylight™ 800 diluted 1:1000 in blocking buffer. Cover plate with aluminum foil to protect the Dylight™ from exposure to light.
- Incubate with secondary antibody for 1 hour at room temperature with gentle shaking.
- Wash by adding 200 µL/well 1X PBS + 0.1% Tween-20 4 times for 5 minutes each at room temperature with gentle shaking.
- After the last wash, gently pipette out any residual liquid and blot plate dry.
- Scan plate.
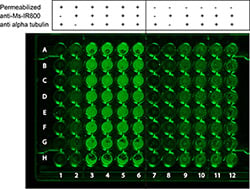
Figure: In-cell Western data showing detection of tubulin in permeabilized Capan-2 cells. A 96-well tissue culture plate was seeded with Capan-2 cells, and after treatment with mouse anti-tubulin and Dylight™ 800 conjugated anti-mouse secondary, alpha-tubulin is detected. Note that detection of tubulin is demonstrated only in the permeabilized cells (columns 3- 6). The dark regions in columns H3-6 is thought to be disruption of the cells by the pipette tips. The legend at top indicates the specific conditions used for each column of cells.