Tips for Multiplex Fluorescent Western Blotting
Fluorescence-based multiplex Western blot is suited for simultaneous detection and quantification of specific proteins in a biological sample. Using a combination of two or three antibodies, fluorescent detection enables simultaneous quantitative analysis of multiple proteins within the same sample on the same blot. The fluorescent dyes—when conjugated to secondary antibodies—offer a variety of benefits over traditional colorimetric and chemiluminescent detection methods. Fluorescent multiplexing reduces complete workflow by minimizing the requirement for multiple blots and allows for a more reliable quantitation of multiple proteins. Other benefits of fluorescent Western blotting include increased sensitivity, excellent signal stability over time, as well as precise quantitative analysis. Multiplex fluorescent Western blotting also reduces or eliminates the need to strip and re-probe. In addition, due to their exceptional photostability, fluorescent dye conjugates can be archived and visualized several times without a decrease in signal; however, using two or three-color detection requires careful selection of primary and secondary antibodies.
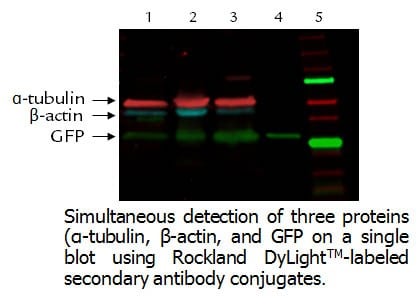
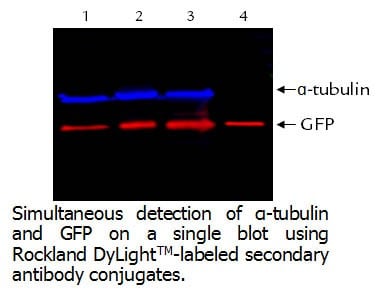
-
Optimizing
Optimize primary and secondary antibody concentrations by incubating the membranes in several dilutions of each antibody and elect the dilution that yields the highest signal-to-background ratio.
-
Choosing a Host
Use primary antibodies from distantly related host species such as mouse, rabbit, or chicken to minimize non-specific binding when using indirect detection. Avoid using primary antibodies derived from mouse and rat together as these species are closely related and may cause cross-reactivity.
For the primary antibodies that are derived from host species (e.g., mouse and chicken), fluorescent dye-IgG secondary antibodies derived from the same host and labeled with different fluorophores must be used (Dylight™ 800 Anti-Mouse (GOAT) and Dylight™ 488 or Dylight™ 649 Anti-Rabbit (GOAT) can be used.)
-
Preliminary Blotting
Before combining primary antibodies in a two- or three-color experiment, always perform separate preliminary blots with each primary antibody to determine the expected banding pattern and possible background bands. The more proteins combined on a blot, the more complex optimization becomes. Single-target detection helps determine the banding pattern of each antibody prior to multiplex analysis.
-
Choosing a Secondary
Use highly cross-adsorbed secondary antibodies. Failure to use highly cross-adsorbed antibodies may result in cross-reactivity.
-
Choosing Conjugates
Use fluorophore conjugates with non-overlapping emission spectra to avoid cross-channel fluorescence or bleed-through. Due to higher wavelength and lower auto-fluorescence, optimal detection of lower abundance targets may be achieved by detecting targets in the 800 channel with normalization in the 649 channel.
-
Choosing a Membrane
To minimize auto-fluorescence, substitute standard PVDF membranes for low-fluorescence PVDF membranes. These membranes increase signal-to-background ratios per channel for transferred proteins, producing bright, clean bands.
-
Choosing Reagents and Buffers
Use high-quality reagents and buffers. Filter sterilize all buffers as particulates in buffers can settle on membranes and create fluorescent artifacts.
-
Running the Dye
Run the dye front sufficiently far away from the expected protein size as the bromophenol blue in loading dye tends to fluoresce.
-
Handling
Use a clean, dust-free, incubation box for each step of the fluorescent Western blotting procedure. To minimize fluorescent artifacts, avoid using inks to mark membranes, don’t handle while wearing powdered nitrile gloves, and make sure to be gentle when handling membranes.
-
Storing
While there’s no risk to photo-bleaching fluorescent-labeled antibodies in working daylight conditions, all fluorescent-labeled antibody stocks should be stored in the dark.