Primary Antibodies
Advance your research with Rockland’s ready-to-use primary antibodies, generated from a variety of hosts and ideal for researching apolipoproteins, apoptosis, epigenetics, the extracellular matrix—to name a few. Our antibodies can recognize and bind with high affinity and specificity to purify, detect, and quantify the antigen.
Recommended RFP and GFP Antibodies
600-401-379
928 References
Applications
WB, ELISA, IHC, IF, FC, EM, FISH, IP, Multiplex, Other
600-101-215
340 References
Applications
WB, ELISA, IHC, IF, FC, EM, FISH, IP, Multiplex, Other, Purification
600-401-215
82 References
Applications
WB, ELISA, IHC, IF, EM, IP, Multiplex, Other, Purification
Featured Primary Antibodies
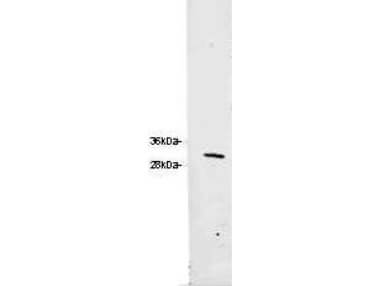
600-401-103-0.1
210 References
Applications
WB, ELISA, IHC, IF, FC, Dot Blot, IP, Multiplex
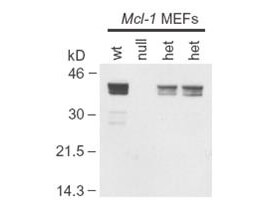
200-301-400
118 References
Applications
WB, ELISA, IHC, IF, FC, Biochemical Assay, ChIP, FISH, IP, Multiplex
200-401-A50
76 References
Applications
WB, ELISA, IHC, IF, FC, FISH, LFA, Multiplex, Other