Immunoassay Oligonucleotide Analysis
Immunoassay Oligonucleotide Analysis
Rockland’s assay development team will work with you to design and execute in-vitro immunoassays for determining the binding characteristics and affinity ranking of your oligonucleotide candidates. We offer methods tailored to your specific oligonucleotide candidates, individual formulations, and analytical goals to provide the critical data you need for accelerated candidate triage.
Analytical Assays for Oligonucleotide Therapeutics
Assessing oligonucleotide drug candidates during early discovery stages can be time-consuming and laborious. To move the best candidate through the pipeline, one must evaluate the absorption, distribution, metabolic, and excretion (ADME) profiles of one or many oligonucleotides for each candidate. In addition, pharmacokinetics (PK)/pharmacodynamic (PD) properties must be understood and immunogenicity studies must be performed.
Although current assays can address these parameters, they come with many disadvantages, such as the need for multiple probes, sequence specificity, and off-target binding for smaller oligonucleotides. Rockland’s proprietary ModDetect™ panels of reagents were developed to solve this challenge by specifically detecting oligonucleotide modifications, such as Phosphorothioate (PS) and 2’-O-Methoxyethyl (MOE), independent of their sequence or location.
Analytical assays, especially immunoassays, provide an alternative approach for collecting insights on PK/PD properties and ADME parameters, while also overcoming limitations of current methods. Rockland can design and run a variety of functional assays to provide critical information about product efficacy, quality, and safety.
Explore our assay options:



ModDetect Analytical Evaluation
Find out if ModDetect will work for your oligonucleotide therapeutic program. Let our team design and execute the in-vitro immunoassays you need to determine the binding characteristics and affinity ranking of ModDetect reagents to your oligonucleotide candidates that contain PS or MOE modifications.
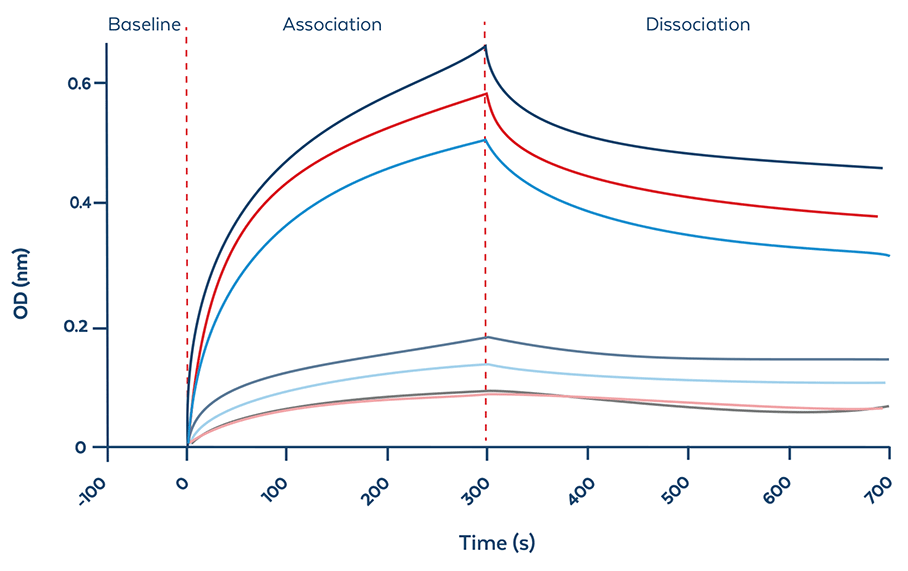
BLI Evaluation
| Parameter | Result |
| Affinity Constant (KD) | 1.25 nM |
| Association Rate (Ka) | 6.25 x 105 M-1s-1 |
| Dissociation Rate (Kd) | 7.81 x 10-4 s-1 |
Figure and table 1. BLI evaluation determines the distinctive binding characteristics of ModDetect reagents against candidate OTx drugs. Data shows representative cycle graph (fitted) showing the baseline, association, and dissociation of ModDetect PS07 and Gapmer. Table shows the summary of calculated binding kinetics of ModDetect PS07 and Gapmer (right).
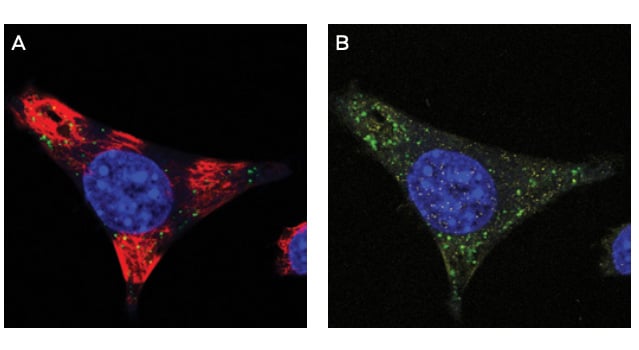
IF Evaluation
Figure 2A-B. IF of ModDetect Reagent PS05 IF of ModDetect Reagent PS05. Mouse glioma cells derived from C57 black mice were cultured and treated with Oligo Tx drug. After fixation with paraformaldehyde, cells were stained with alpha-tubulin (red) and ModDetect reagent PS05 (green). Reagent PS05 was used at a 1:2000 dilution. Punctate cytoplasmic staining is consistent with endosomal storage of ASO within the cell, as expected for this Oligo Tx drug. Vehicle-only treated cells showed no staining (not shown).
ELISA Evaluation
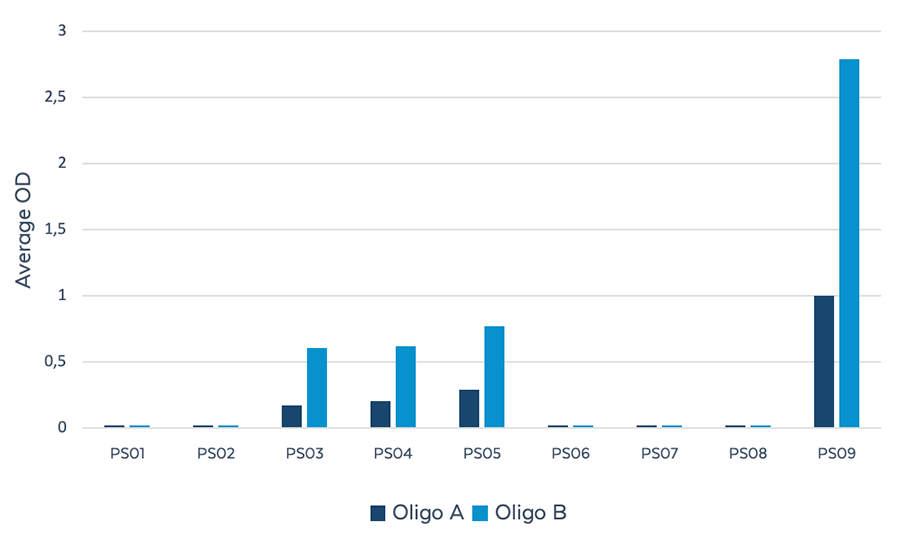
Figure 3. Reactivity of PS reagents against client-provided candidate OTx drugs using indirect ELISA. Candidate drugs (A and B ) containing different configurations and number of PS modifications were screened against the ModDetect PS Panel. Candidates A and B showed the most reactivity to PS03, PS04, PS05, and PS09.
Sandwich ELISA Evaluation
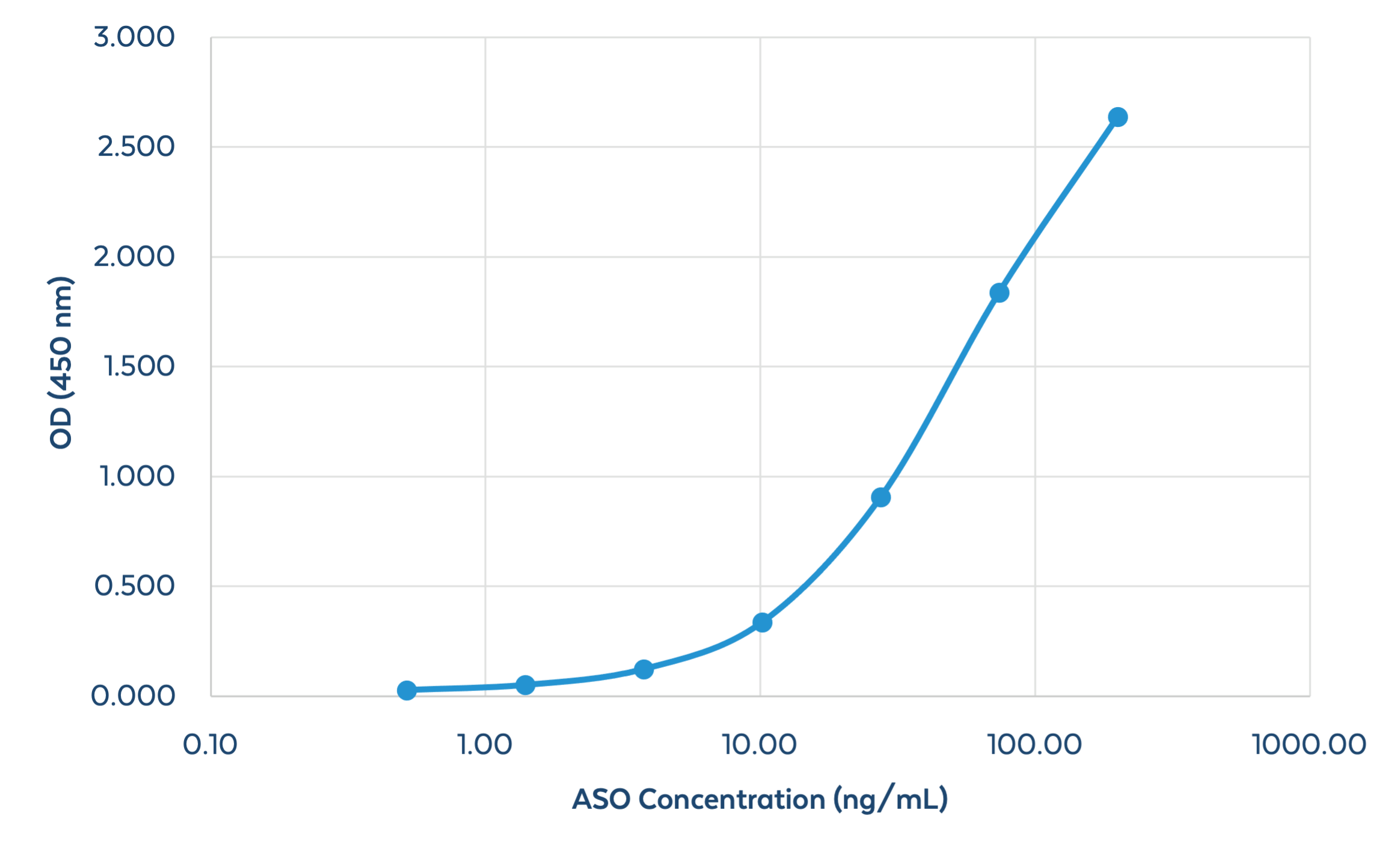
Figure 4. Quantitation of an ASO using Sandwich ELISA. A method for the quantification of PS-modified oligonucleotides (in buffer) using a Sandwich ELISA has been developed for use. A 100% PS-modified ASO 29mer was detected using the ModDetect PS Panel. The range of detection for the 29mer was determined to be 0.5 – 200 ng/mL. The basis of this method can be used to quantify amounts of OTx drugs in biological samples such as serum, blood, homogenized tissue, and urine.
Let us do the heavy lifting:
Reduce workload when internal resources are limited
Save time to focus on oligonucleotide-specific challenges
Gain comprehensive data and recommendations from immunoassay experts
Accelerate oligonucleotide candidate triage
Interested in immunoassay oligonucleotide analysis?
Talk with us to find out how we can help move your research forward.